We provide a full suite of complementary solutions to boost value for your business.
Our patented, encryption-based system ensures scalability and code uniqueness. Highly adaptable, it can be adopted as a complete integrated solution, or as a software suite, easily integrated with pre-existing hardware. Fully documented and built to ensure compliance with current and changing mandates across the world.
You can efficiently prevent counterfeiting and track inventory at every stage – from the manufacturing stage to final delivery or purchase by a customer. Moreover, use insights to improve sales and identify distribution inefficiencies to be addressed.

We have implemented our solutions across hundreds of packaging lines in India and beyond, and take pride in our innovative solutions that can facilitate robust, cost-efficient serialization and aggregation on most bottle, blister, strip or other lines with minimal downtime or impact to production speeds and rejection rates.
Our patented, encryption-based psID® system generates random, unique, alphanumeric QR or GS1 2D Datamatrix codes that can be easily scanned by consumers to verify products. Not only does this help in anti-counterfeiting, cut significant losses but also helps build trust and brand loyalty.
Our loyalty platform can be used to create multi-channel programs for your customers, retailers and influencers. Use smart technology to effectively connect with them and boost your return on investment and sales.
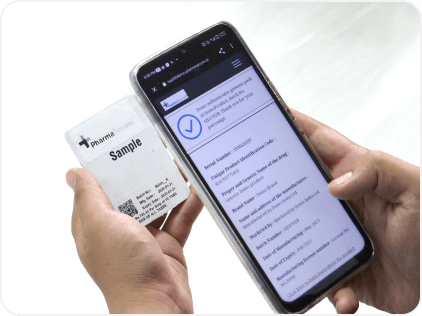
Effective August 1, 2023, the Top 300 pharmaceutical brands in India are required to print QR codes/barcodes on their products that when scanned verify the product and provide additional details. PharmaSecure provides a complete hardware-and-software solution in line with GS1 standards to help you meet the requirements seamlessly and efficiently.

Production Line Installations

Products Secured

Years of Experience

We have over 15 years of experience with pharmaceutical clients – some of the largest pharmaceutical companies rely on us for their regulatory serialisation and brand protection needs. Moreover, our unrivalled after-sales service helps clients seamlessly carry on their operations and manufacturing.

In an ever-evolving regulatory environment, it’s important for companies to stay future-fit. This is especially true for companies exporting across the globe. So, to ensure complete regulatory compliance for our clients, we support them for current as well as upcoming mandates.

Till date PharmaSecure has issued and applied 6 billion codes, more than 550 packaging line integrations with over 20 OEMs. Our patented system ensures scalability, code uniqueness and best-in-class security. We provide database-less technology that eliminates the security risks of conventional solutions.

With PharmaSecure, you can safely expect minimal disruption to your existing manufacturing process and negligible downtime of manufacturing lines. Our platform effortlessly integrates with industry-standard printers and verification systems.